Optimized iphone 5s cg glue dispensing cycle time by 37 while maintaining overall push force and glue overflow. 1 product development engineer.
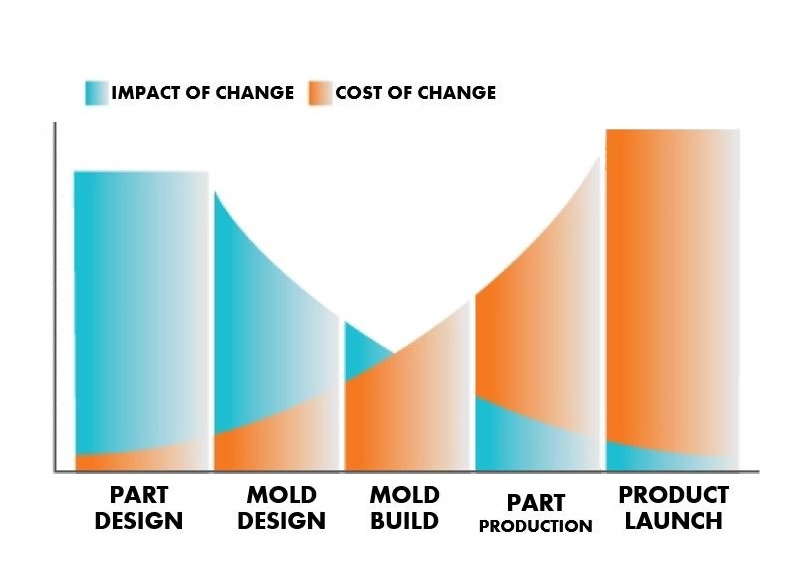
What Is Design For Manufacturing Or Dfm
Medical device product development engineer resume. Executing quality engineering related activities including problem solving and product and process quality improvement. Apply to product development engineer product engineer validation engineer and more. Qualityvalidation engineer medical devices professional summary dynamic and experience leader as a processvalidation quality manufacturing engineer in the manufacturing industry with strong experience in process improvement product development and implementation of lean with six sigma tool. The clinical development engineer cde uses their in depth knowledge of medical technologies to work with design engineers and clinicians to define develop and validate the clinical function of new products within robotic platforms. Medical devices design development engineer march 2011 to current mbmed. Biomedical engineers research and develop new medical technologies.
Research design develop specify analyze simulate prototype document code test and release to manufacturing the new medical device. Interface with the idm device platform advisors on the device strategy and technical agenda to lead applicable projects. Apply to product development engineer electrical engineer senior product development engineer and more. Example resumes for this position highlight such skills as providing mechanical design fabrication assembling testing and documentation of the components modules and complete medical devices. A minimum of 5 years of business formation experience with new product development in the medical device market including a minimum of 3 years of demonstrated management experience experience negotiating term sheets valued more than 10 million with top venture capital firms and an understanding of the technology transfer licensing process to startup companies is a plus. Participate in design and manufacturability reviews.
Improved iphone 5c internal assembly yield by 10 by analyzing cpk report determining the root cause and proposing modification on assembly fixture. Medical device engineer ii. Bachelor of science with nine to thirteen years of experience in the pharmaceutical biotech device or drug delivery industry or masters of science with seven to eleven years of experience in the pharmaceutical biotech device or drug delivery industry or phd. Project manager design and development of medical devices including. Bachelors degree in engineering mechanical or electrical engineer preferred minimum of two years experience in medical device manufacturing. With three to six years of experience in the pharmaceutical biotech device or drug delivery industry.


:max_bytes(150000):strip_icc()/2060124v1-5be328a046e0fb002621fdae.png)